-
Notifications
You must be signed in to change notification settings - Fork 9
Example Tutorial #06: Rosa rugosa invaded coastal grassland communities in Belgium
Shawn P. Serbin edited this page Jun 19, 2024
·
1 revision
Spectra-trait PLSR example using leaf-level spectra and leaf nitrogen content (Narea, g/m2) data from 36 species growing in Rosa rugosa invaded coastal grassland communities in Belgium
Shawn P. Serbin, Julien Lamour, & Jeremiah Anderson 2024-06-19
This is an R Markdown Notebook to illustrate how to retrieve a dataset from the EcoSIS spectral database, choose the “optimal” number of plsr components, and fit a plsr model for leaf nitrogen content (Narea, g/m2)
DOI: https://doi.org/10.1111/1365-2745.13389
list.of.packages <- c("pls","dplyr","here","plotrix","ggplot2","gridExtra","spectratrait")
invisible(lapply(list.of.packages, library, character.only = TRUE))## Warning: package 'pls' was built under R version 4.3.1
##
## Attaching package: 'pls'
## The following object is masked from 'package:stats':
##
## loadings
## Warning: package 'dplyr' was built under R version 4.3.1
##
## Attaching package: 'dplyr'
## The following objects are masked from 'package:stats':
##
## filter, lag
## The following objects are masked from 'package:base':
##
## intersect, setdiff, setequal, union
## here() starts at /Users/sserbin/Library/CloudStorage/OneDrive-NASA/Data/Github/spectratrait
## Warning: package 'plotrix' was built under R version 4.3.1
## Warning: package 'ggplot2' was built under R version 4.3.1
##
## Attaching package: 'gridExtra'
## The following object is masked from 'package:dplyr':
##
## combine
### Setup options
# Script options
pls::pls.options(plsralg = "oscorespls")
pls::pls.options("plsralg")## $plsralg
## [1] "oscorespls"
# Default par options
opar <- par(no.readonly = T)
# What is the target variable?
inVar <- "Narea_g_m2"
# What is the source dataset from EcoSIS?
ecosis_id <- "9db4c5a2-7eac-4e1e-8859-009233648e89"
# Specify output directory, output_dir
# Options:
# tempdir - use a OS-specified temporary directory
# user defined PATH - e.g. "~/scratch/PLSR"
output_dir <- "tempdir"## [1] "/private/var/folders/th/fpt_z3417gn8xgply92pvy6r0000gq/T/RtmpP2TT60"
print(paste0("Output directory: ",getwd())) # check wd## [1] "Output directory: /Users/sserbin/Library/CloudStorage/OneDrive-NASA/Data/Github/spectratrait/vignettes"
dat_raw <- spectratrait::get_ecosis_data(ecosis_id = ecosis_id)## [1] "**** Downloading Ecosis data ****"
## Downloading data...
## Rows: 256 Columns: 2164
## ── Column specification ────────────────────────────────────────────────────────
## Delimiter: ","
## chr (4): Latin Species, ids, plot code, species code
## dbl (2160): Cw/EWT (cm3/cm2), Leaf area (mm2), Leaf calcium content per leaf...
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.
## Download complete!
head(dat_raw)## # A tibble: 6 × 2,164
## `Cw/EWT (cm3/cm2)` `Latin Species` `Leaf area (mm2)` Leaf calcium content…¹
## <dbl> <chr> <dbl> <dbl>
## 1 0.00887 Arrhenatherum ela… 696. 0.0291
## 2 0.00824 Bromus sterilis 447. 0.0230
## 3 0.0280 Jacobaea vulgaris 2418. 0.0950
## 4 0.0106 Rubus caesius 5719. 0.0700
## 5 0.00851 Arrhenatherum ela… 671. 0.0286
## 6 0.0153 Crepis capillaris 1401. 0.0470
## # ℹ abbreviated name: ¹`Leaf calcium content per leaf area (mg/mm2)`
## # ℹ 2,160 more variables:
## # `Leaf magnesium content per leaf area (mg/mm2)` <dbl>,
## # `Leaf mass per area (g/cm2)` <dbl>,
## # `Leaf nitrogen content per leaf area (mg/mm2)` <dbl>,
## # `Leaf phosphorus content per leaf area (mg/mm2)` <dbl>,
## # `Leaf potassium content per leaf area (mg/mm2)` <dbl>, …
names(dat_raw)[1:40]## [1] "Cw/EWT (cm3/cm2)"
## [2] "Latin Species"
## [3] "Leaf area (mm2)"
## [4] "Leaf calcium content per leaf area (mg/mm2)"
## [5] "Leaf magnesium content per leaf area (mg/mm2)"
## [6] "Leaf mass per area (g/cm2)"
## [7] "Leaf nitrogen content per leaf area (mg/mm2)"
## [8] "Leaf phosphorus content per leaf area (mg/mm2)"
## [9] "Leaf potassium content per leaf area (mg/mm2)"
## [10] "Plant height vegetative (cm)"
## [11] "ids"
## [12] "plot code"
## [13] "species code"
## [14] "350"
## [15] "351"
## [16] "352"
## [17] "353"
## [18] "354"
## [19] "355"
## [20] "356"
## [21] "357"
## [22] "358"
## [23] "359"
## [24] "360"
## [25] "361"
## [26] "362"
## [27] "363"
## [28] "364"
## [29] "365"
## [30] "366"
## [31] "367"
## [32] "368"
## [33] "369"
## [34] "370"
## [35] "371"
## [36] "372"
## [37] "373"
## [38] "374"
## [39] "375"
## [40] "376"
### Create plsr dataset
Start.wave <- 500
End.wave <- 2400
wv <- seq(Start.wave,End.wave,1)
Spectra <- as.matrix(dat_raw[,names(dat_raw) %in% wv])
colnames(Spectra) <- c(paste0("Wave_",wv))
sample_info <- dat_raw[,names(dat_raw) %notin% seq(350,2500,1)]
head(sample_info)## # A tibble: 6 × 13
## `Cw/EWT (cm3/cm2)` `Latin Species` `Leaf area (mm2)` Leaf calcium content…¹
## <dbl> <chr> <dbl> <dbl>
## 1 0.00887 Arrhenatherum ela… 696. 0.0291
## 2 0.00824 Bromus sterilis 447. 0.0230
## 3 0.0280 Jacobaea vulgaris 2418. 0.0950
## 4 0.0106 Rubus caesius 5719. 0.0700
## 5 0.00851 Arrhenatherum ela… 671. 0.0286
## 6 0.0153 Crepis capillaris 1401. 0.0470
## # ℹ abbreviated name: ¹`Leaf calcium content per leaf area (mg/mm2)`
## # ℹ 9 more variables: `Leaf magnesium content per leaf area (mg/mm2)` <dbl>,
## # `Leaf mass per area (g/cm2)` <dbl>,
## # `Leaf nitrogen content per leaf area (mg/mm2)` <dbl>,
## # `Leaf phosphorus content per leaf area (mg/mm2)` <dbl>,
## # `Leaf potassium content per leaf area (mg/mm2)` <dbl>,
## # `Plant height vegetative (cm)` <dbl>, ids <chr>, `plot code` <chr>, …
sample_info2 <- sample_info %>%
select(Plant_Species=`Latin Species`,Species_Code=`species code`,Plot=`plot code`,
Narea_mg_mm2=`Leaf nitrogen content per leaf area (mg/mm2)`)
sample_info2 <- sample_info2 %>%
# mutate(Narea_g_m2=Narea_mg_mm2*(0.001/1e-6)) # based on orig units should be this but conversion wrong
mutate(Narea_g_m2=Narea_mg_mm2*100) # this assumes orig units were g/mm2 or mg/cm2
head(sample_info2)## # A tibble: 6 × 5
## Plant_Species Species_Code Plot Narea_mg_mm2 Narea_g_m2
## <chr> <chr> <chr> <dbl> <dbl>
## 1 Arrhenatherum elatius Arrela DC1 0.0126 1.26
## 2 Bromus sterilis Broste DC1 0.00682 0.682
## 3 Jacobaea vulgaris Jacvul DC1 0.0102 1.02
## 4 Rubus caesius Rubcae DC1 0.0121 1.21
## 5 Arrhenatherum elatius Arrela DC2 0.0117 1.17
## 6 Crepis capillaris Creves DC2 0.00877 0.877
plsr_data <- data.frame(sample_info2,Spectra)
rm(sample_info,sample_info2,Spectra)#### End user needs to do what's appropriate for their data. This may be an iterative process.
# Keep only complete rows of inVar and spec data before fitting
plsr_data <- plsr_data[complete.cases(plsr_data[,names(plsr_data) %in%
c(inVar,paste0("Wave_",wv))]),]### Create cal/val datasets
## Make a stratified random sampling in the strata USDA_Species_Code and Domain
method <- "dplyr" #base/dplyr
# base R - a bit slow
# dplyr - much faster
split_data <- spectratrait::create_data_split(dataset=plsr_data, approach=method, split_seed=1245565,
prop=0.8, group_variables="Species_Code")
names(split_data)## [1] "cal_data" "val_data"
cal.plsr.data <- split_data$cal_data
head(cal.plsr.data)[1:8]## Plant_Species Species_Code Plot Narea_mg_mm2 Narea_g_m2 Wave_500
## 1 Ammophila arenaria Ammare ZC3 0.03240495 3.240495 0.130885
## 2 Ammophila arenaria Ammare MC2 0.02806279 2.806279 0.135785
## 3 Ammophila arenaria Ammare ZC1 0.02041612 2.041612 0.147665
## 4 Ammophila arenaria Ammare MC1 0.02426549 2.426549 0.142765
## 5 Ammophila arenaria Ammare WC3 0.02807281 2.807281 0.151750
## 6 Ammophila arenaria Ammare WR3 0.02286678 2.286678 0.150850
## Wave_501 Wave_502
## 1 0.13175 0.132750
## 2 0.13685 0.138150
## 3 0.14910 0.150330
## 4 0.14390 0.145200
## 5 0.15275 0.154150
## 6 0.15185 0.152815
val.plsr.data <- split_data$val_data
head(val.plsr.data)[1:8]## Plant_Species Species_Code Plot Narea_mg_mm2 Narea_g_m2 Wave_500
## 1 Arrhenatherum elatius Arrela DC1 0.01261440 1.261440 0.07066700
## 4 Rubus caesius Rubcae DC1 0.01208978 1.208978 0.04144907
## 8 Jacobaea vulgaris Jacvul DC2 0.01185197 1.185197 0.05563100
## 11 Carex arenaria Carare DC3 0.02103830 2.103830 0.11588500
## 14 Jacobaea vulgaris Jacvul DC3 0.01121247 1.121247 0.06029327
## 19 Oenothera glazioviana Oengla DC4 0.01444293 1.444293 0.07391700
## Wave_501 Wave_502
## 1 0.07160000 0.0725330
## 4 0.04197333 0.0426356
## 8 0.05622143 0.0569690
## 11 0.11705000 0.1184500
## 14 0.06112000 0.0620312
## 19 0.07515000 0.0765500
rm(split_data)
# Datasets:
print(paste("Cal observations: ",dim(cal.plsr.data)[1],sep=""))## [1] "Cal observations: 183"
print(paste("Val observations: ",dim(val.plsr.data)[1],sep=""))## [1] "Val observations: 73"
cal_hist_plot <- ggplot(data = cal.plsr.data,
aes(x = cal.plsr.data[,paste0(inVar)])) +
geom_histogram(fill=I("grey50"),col=I("black"),alpha=I(.7)) +
labs(title=paste0("Calibration Histogram for ",inVar), x = paste0(inVar),
y = "Count")
val_hist_plot <- ggplot(data = val.plsr.data,
aes(x = val.plsr.data[,paste0(inVar)])) +
geom_histogram(fill=I("grey50"),col=I("black"),alpha=I(.7)) +
labs(title=paste0("Validation Histogram for ",inVar), x = paste0(inVar),
y = "Count")
histograms <- grid.arrange(cal_hist_plot, val_hist_plot, ncol=2)## `stat_bin()` using `bins = 30`. Pick better value with `binwidth`.
## `stat_bin()` using `bins = 30`. Pick better value with `binwidth`.

ggsave(filename = file.path(outdir,paste0(inVar,"_Cal_Val_Histograms.png")), plot = histograms,
device="png", width = 30,
height = 12, units = "cm",
dpi = 300)
# output cal/val data
write.csv(cal.plsr.data,file=file.path(outdir,paste0(inVar,'_Cal_PLSR_Dataset.csv')),
row.names=FALSE)
write.csv(val.plsr.data,file=file.path(outdir,paste0(inVar,'_Val_PLSR_Dataset.csv')),
row.names=FALSE)### Format PLSR data for model fitting
cal_spec <- as.matrix(cal.plsr.data[, which(names(cal.plsr.data) %in% paste0("Wave_",wv))])
cal.plsr.data <- data.frame(cal.plsr.data[, which(names(cal.plsr.data) %notin% paste0("Wave_",wv))],
Spectra=I(cal_spec))
head(cal.plsr.data)[1:5]## Plant_Species Species_Code Plot Narea_mg_mm2 Narea_g_m2
## 1 Ammophila arenaria Ammare ZC3 0.03240495 3.240495
## 2 Ammophila arenaria Ammare MC2 0.02806279 2.806279
## 3 Ammophila arenaria Ammare ZC1 0.02041612 2.041612
## 4 Ammophila arenaria Ammare MC1 0.02426549 2.426549
## 5 Ammophila arenaria Ammare WC3 0.02807281 2.807281
## 6 Ammophila arenaria Ammare WR3 0.02286678 2.286678
val_spec <- as.matrix(val.plsr.data[, which(names(val.plsr.data) %in% paste0("Wave_",wv))])
val.plsr.data <- data.frame(val.plsr.data[, which(names(val.plsr.data) %notin% paste0("Wave_",wv))],
Spectra=I(val_spec))
head(val.plsr.data)[1:5]## Plant_Species Species_Code Plot Narea_mg_mm2 Narea_g_m2
## 1 Arrhenatherum elatius Arrela DC1 0.01261440 1.261440
## 4 Rubus caesius Rubcae DC1 0.01208978 1.208978
## 8 Jacobaea vulgaris Jacvul DC2 0.01185197 1.185197
## 11 Carex arenaria Carare DC3 0.02103830 2.103830
## 14 Jacobaea vulgaris Jacvul DC3 0.01121247 1.121247
## 19 Oenothera glazioviana Oengla DC4 0.01444293 1.444293
par(mfrow=c(1,2)) # B, L, T, R
spectratrait::f.plot.spec(Z=cal.plsr.data$Spectra,wv=wv,plot_label="Calibration")
spectratrait::f.plot.spec(Z=val.plsr.data$Spectra,wv=wv,plot_label="Validation")
dev.copy(png,file.path(outdir,paste0(inVar,'_Cal_Val_Spectra.png')),
height=2500,width=4900, res=340)## quartz_off_screen
## 3
dev.off();## quartz_off_screen
## 2
par(mfrow=c(1,1))### Use permutation to determine the optimal number of components
if(grepl("Windows", sessionInfo()$running)){
pls.options(parallel = NULL)
} else {
pls.options(parallel = parallel::detectCores()-1)
}
method <- "pls" #pls, firstPlateau, firstMin
random_seed <- 1245565
seg <- 50
maxComps <- 16
iterations <- 80
prop <- 0.70
if (method=="pls") {
# pls package approach - faster but estimates more components....
nComps <- spectratrait::find_optimal_components(dataset=cal.plsr.data, targetVariable=inVar,
method=method,
maxComps=maxComps, seg=seg,
random_seed=random_seed)
print(paste0("*** Optimal number of components: ", nComps))
} else {
nComps <- spectratrait::find_optimal_components(dataset=cal.plsr.data, targetVariable=inVar,
method=method,
maxComps=maxComps, iterations=iterations,
seg=seg, prop=prop,
random_seed=random_seed)
}## [1] "*** Identifying optimal number of PLSR components ***"
## [1] "*** Running PLS permutation test ***"

## [1] "*** Optimal number of components: 10"
dev.copy(png,file.path(outdir,paste0(paste0(inVar,"_PLSR_Component_Selection.png"))),
height=2800, width=3400, res=340)## quartz_off_screen
## 3
dev.off();## quartz_off_screen
## 2
plsr.out <- plsr(as.formula(paste(inVar,"~","Spectra")),scale=FALSE,ncomp=nComps,validation="LOO",
trace=FALSE,data=cal.plsr.data)
fit <- plsr.out$fitted.values[,1,nComps]
pls.options(parallel = NULL)
# External validation fit stats
par(mfrow=c(1,2)) # B, L, T, R
pls::RMSEP(plsr.out, newdata = val.plsr.data)## (Intercept) 1 comps 2 comps 3 comps 4 comps 5 comps
## 0.5594 0.6034 0.5448 0.3842 0.3481 0.3027
## 6 comps 7 comps 8 comps 9 comps 10 comps
## 0.2429 0.2268 0.2852 0.2818 0.2780
plot(pls::RMSEP(plsr.out,estimate=c("test"),newdata = val.plsr.data), main="MODEL RMSEP",
xlab="Number of Components",ylab="Model Validation RMSEP",lty=1,col="black",cex=1.5,lwd=2)
box(lwd=2.2)
pls::R2(plsr.out, newdata = val.plsr.data)## (Intercept) 1 comps 2 comps 3 comps 4 comps 5 comps
## -0.007544 -0.172296 0.044153 0.524579 0.609920 0.704963
## 6 comps 7 comps 8 comps 9 comps 10 comps
## 0.809962 0.834383 0.738093 0.744325 0.751224
plot(pls::R2(plsr.out,estimate=c("test"),newdata = val.plsr.data), main="MODEL R2",
xlab="Number of Components",ylab="Model Validation R2",lty=1,col="black",cex=1.5,lwd=2)
box(lwd=2.2)
dev.copy(png,file.path(outdir,paste0(paste0(inVar,"_Validation_RMSEP_R2_by_Component.png"))),
height=2800, width=4800, res=340)## quartz_off_screen
## 3
dev.off();## quartz_off_screen
## 2
par(opar)#calibration
cal.plsr.output <- data.frame(cal.plsr.data[, which(names(cal.plsr.data) %notin% "Spectra")],
PLSR_Predicted=fit,
PLSR_CV_Predicted=as.vector(plsr.out$validation$pred[,,nComps]))
cal.plsr.output <- cal.plsr.output %>%
mutate(PLSR_CV_Residuals = PLSR_CV_Predicted-get(inVar))
head(cal.plsr.output)## Plant_Species Species_Code Plot Narea_mg_mm2 Narea_g_m2 PLSR_Predicted
## 1 Ammophila arenaria Ammare ZC3 0.03240495 3.240495 2.672029
## 2 Ammophila arenaria Ammare MC2 0.02806279 2.806279 2.651863
## 3 Ammophila arenaria Ammare ZC1 0.02041612 2.041612 2.178056
## 4 Ammophila arenaria Ammare MC1 0.02426549 2.426549 2.412013
## 5 Ammophila arenaria Ammare WC3 0.02807281 2.807281 2.452711
## 6 Ammophila arenaria Ammare WR3 0.02286678 2.286678 2.792340
## PLSR_CV_Predicted PLSR_CV_Residuals
## 1 2.598245 -0.642250440
## 2 2.652066 -0.154212969
## 3 2.200588 0.158975634
## 4 2.435784 0.009234491
## 5 2.384049 -0.423231444
## 6 2.943186 0.656508493
cal.R2 <- round(pls::R2(plsr.out,intercept=F)[[1]][nComps],2)
cal.RMSEP <- round(sqrt(mean(cal.plsr.output$PLSR_CV_Residuals^2)),2)
val.plsr.output <- data.frame(val.plsr.data[, which(names(val.plsr.data) %notin% "Spectra")],
PLSR_Predicted=as.vector(predict(plsr.out,
newdata = val.plsr.data,
ncomp=nComps, type="response")[,,1]))
val.plsr.output <- val.plsr.output %>%
mutate(PLSR_Residuals = PLSR_Predicted-get(inVar))
head(val.plsr.output)## Plant_Species Species_Code Plot Narea_mg_mm2 Narea_g_m2
## 1 Arrhenatherum elatius Arrela DC1 0.01261440 1.261440
## 4 Rubus caesius Rubcae DC1 0.01208978 1.208978
## 8 Jacobaea vulgaris Jacvul DC2 0.01185197 1.185197
## 11 Carex arenaria Carare DC3 0.02103830 2.103830
## 14 Jacobaea vulgaris Jacvul DC3 0.01121247 1.121247
## 19 Oenothera glazioviana Oengla DC4 0.01444293 1.444293
## PLSR_Predicted PLSR_Residuals
## 1 1.340135 0.07869548
## 4 1.288026 0.07904830
## 8 1.155840 -0.02935675
## 11 2.014712 -0.08911757
## 14 1.328742 0.20749565
## 19 1.534162 0.08986811
val.R2 <- round(pls::R2(plsr.out,newdata=val.plsr.data,intercept=F)[[1]][nComps],2)
val.RMSEP <- round(sqrt(mean(val.plsr.output$PLSR_Residuals^2)),2)
rng_quant <- quantile(cal.plsr.output[,inVar], probs = c(0.001, 0.999))
cal_scatter_plot <- ggplot(cal.plsr.output, aes(x=PLSR_CV_Predicted, y=get(inVar))) +
theme_bw() + geom_point() + geom_abline(intercept = 0, slope = 1, color="dark grey",
linetype="dashed", linewidth=1.5) +
xlim(rng_quant[1], rng_quant[2]) +
ylim(rng_quant[1], rng_quant[2]) +
labs(x=paste0("Predicted ", paste(inVar), " (units)"),
y=paste0("Observed ", paste(inVar), " (units)"),
title=paste0("Calibration: ", paste0("Rsq = ", cal.R2), "; ", paste0("RMSEP = ",
cal.RMSEP))) +
theme(axis.text=element_text(size=18), legend.position="none",
axis.title=element_text(size=20, face="bold"),
axis.text.x = element_text(angle = 0,vjust = 0.5),
panel.border = element_rect(linetype = "solid", fill = NA, linewidth=1.5))
cal_resid_histogram <- ggplot(cal.plsr.output, aes(x=PLSR_CV_Residuals)) +
geom_histogram(alpha=.5, position="identity") +
geom_vline(xintercept = 0, color="black",
linetype="dashed", linewidth=1) + theme_bw() +
theme(axis.text=element_text(size=18), legend.position="none",
axis.title=element_text(size=20, face="bold"),
axis.text.x = element_text(angle = 0,vjust = 0.5),
panel.border = element_rect(linetype = "solid", fill = NA, linewidth=1.5))
rng_quant <- quantile(val.plsr.output[,inVar], probs = c(0.001, 0.999))
val_scatter_plot <- ggplot(val.plsr.output, aes(x=PLSR_Predicted, y=get(inVar))) +
theme_bw() + geom_point() + geom_abline(intercept = 0, slope = 1, color="dark grey",
linetype="dashed", linewidth=1.5) +
xlim(rng_quant[1], rng_quant[2]) +
ylim(rng_quant[1], rng_quant[2]) +
labs(x=paste0("Predicted ", paste(inVar), " (units)"),
y=paste0("Observed ", paste(inVar), " (units)"),
title=paste0("Validation: ", paste0("Rsq = ", val.R2), "; ", paste0("RMSEP = ",
val.RMSEP))) +
theme(axis.text=element_text(size=18), legend.position="none",
axis.title=element_text(size=20, face="bold"),
axis.text.x = element_text(angle = 0,vjust = 0.5),
panel.border = element_rect(linetype = "solid", fill = NA, linewidth=1.5))
val_resid_histogram <- ggplot(val.plsr.output, aes(x=PLSR_Residuals)) +
geom_histogram(alpha=.5, position="identity") +
geom_vline(xintercept = 0, color="black",
linetype="dashed", linewidth=1) + theme_bw() +
theme(axis.text=element_text(size=18), legend.position="none",
axis.title=element_text(size=20, face="bold"),
axis.text.x = element_text(angle = 0,vjust = 0.5),
panel.border = element_rect(linetype = "solid", fill = NA, linewidth=1.5))
# plot cal/val side-by-side
scatterplots <- grid.arrange(cal_scatter_plot, val_scatter_plot, cal_resid_histogram,
val_resid_histogram, nrow=2,ncol=2)## Warning: Removed 2 rows containing missing values or values outside the scale range
## (`geom_point()`).
## Removed 2 rows containing missing values or values outside the scale range
## (`geom_point()`).
## `stat_bin()` using `bins = 30`. Pick better value with `binwidth`.
## `stat_bin()` using `bins = 30`. Pick better value with `binwidth`.
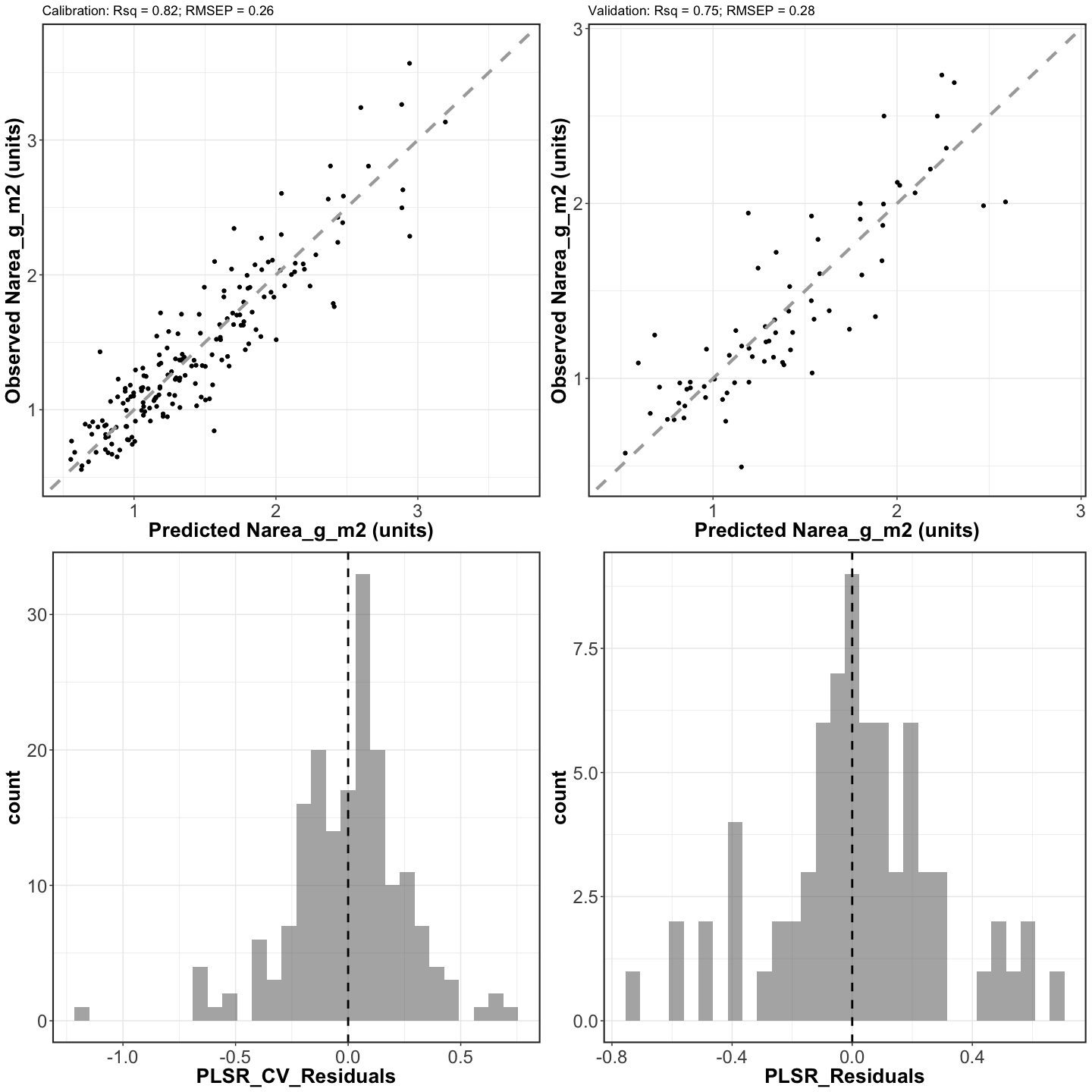
ggsave(filename = file.path(outdir,paste0(inVar,"_Cal_Val_Scatterplots.png")),
plot = scatterplots, device="png",
width = 32,
height = 30, units = "cm",
dpi = 300)vips <- spectratrait::VIP(plsr.out)[nComps,]
par(mfrow=c(2,1))
plot(plsr.out, plottype = "coef",xlab="Wavelength (nm)",
ylab="Regression coefficients",legendpos = "bottomright",
ncomp=nComps,lwd=2)
box(lwd=2.2)
plot(seq(Start.wave,End.wave,1),vips,xlab="Wavelength (nm)",ylab="VIP",cex=0.01)
lines(seq(Start.wave,End.wave,1),vips,lwd=3)
abline(h=0.8,lty=2,col="dark grey")
box(lwd=2.2)
dev.copy(png,file.path(outdir,paste0(inVar,'_Coefficient_VIP_plot.png')),
height=3100, width=4100, res=340)## quartz_off_screen
## 3
dev.off();## quartz_off_screen
## 2
if(grepl("Windows", sessionInfo()$running)){
pls.options(parallel =NULL)
} else {
pls.options(parallel = parallel::detectCores()-1)
}
jk.plsr.out <- pls::plsr(as.formula(paste(inVar,"~","Spectra")), scale=FALSE,
center=TRUE, ncomp=nComps, validation="LOO", trace=FALSE,
jackknife=TRUE,
data=cal.plsr.data)
pls.options(parallel = NULL)
Jackknife_coef <- spectratrait::f.coef.valid(plsr.out = jk.plsr.out, data_plsr = cal.plsr.data,
ncomp = nComps, inVar=inVar)
Jackknife_intercept <- Jackknife_coef[1,,,]
Jackknife_coef <- Jackknife_coef[2:dim(Jackknife_coef)[1],,,]
interval <- c(0.025,0.975)
Jackknife_Pred <- val.plsr.data$Spectra %*% Jackknife_coef +
matrix(rep(Jackknife_intercept, length(val.plsr.data[,inVar])), byrow=TRUE,
ncol=length(Jackknife_intercept))
Interval_Conf <- apply(X = Jackknife_Pred, MARGIN = 1, FUN = quantile,
probs=c(interval[1], interval[2]))
sd_mean <- apply(X = Jackknife_Pred, MARGIN = 1, FUN =sd)
sd_res <- sd(val.plsr.output$PLSR_Residuals)
sd_tot <- sqrt(sd_mean^2+sd_res^2)
val.plsr.output$LCI <- Interval_Conf[1,]
val.plsr.output$UCI <- Interval_Conf[2,]
val.plsr.output$LPI <- val.plsr.output$PLSR_Predicted-1.96*sd_tot
val.plsr.output$UPI <- val.plsr.output$PLSR_Predicted+1.96*sd_tot
head(val.plsr.output)## Plant_Species Species_Code Plot Narea_mg_mm2 Narea_g_m2
## 1 Arrhenatherum elatius Arrela DC1 0.01261440 1.261440
## 4 Rubus caesius Rubcae DC1 0.01208978 1.208978
## 8 Jacobaea vulgaris Jacvul DC2 0.01185197 1.185197
## 11 Carex arenaria Carare DC3 0.02103830 2.103830
## 14 Jacobaea vulgaris Jacvul DC3 0.01121247 1.121247
## 19 Oenothera glazioviana Oengla DC4 0.01444293 1.444293
## PLSR_Predicted PLSR_Residuals LCI UCI LPI UPI
## 1 1.340135 0.07869548 1.298260 1.346986 0.7916762 1.888595
## 4 1.288026 0.07904830 1.262110 1.297939 0.7397937 1.836258
## 8 1.155840 -0.02935675 1.113678 1.172006 0.6072413 1.704439
## 11 2.014712 -0.08911757 1.936508 2.020049 1.4654399 2.563985
## 14 1.328742 0.20749565 1.298485 1.333454 0.7804978 1.876987
## 19 1.534162 0.08986811 1.522672 1.550848 0.9859820 2.082341
val.plsr.output$LPI <- val.plsr.output$PLSR_Predicted-1.96*sd_tot
val.plsr.output$UPI <- val.plsr.output$PLSR_Predicted+1.96*sd_tot
head(val.plsr.output)## Plant_Species Species_Code Plot Narea_mg_mm2 Narea_g_m2
## 1 Arrhenatherum elatius Arrela DC1 0.01261440 1.261440
## 4 Rubus caesius Rubcae DC1 0.01208978 1.208978
## 8 Jacobaea vulgaris Jacvul DC2 0.01185197 1.185197
## 11 Carex arenaria Carare DC3 0.02103830 2.103830
## 14 Jacobaea vulgaris Jacvul DC3 0.01121247 1.121247
## 19 Oenothera glazioviana Oengla DC4 0.01444293 1.444293
## PLSR_Predicted PLSR_Residuals LCI UCI LPI UPI
## 1 1.340135 0.07869548 1.298260 1.346986 0.7916762 1.888595
## 4 1.288026 0.07904830 1.262110 1.297939 0.7397937 1.836258
## 8 1.155840 -0.02935675 1.113678 1.172006 0.6072413 1.704439
## 11 2.014712 -0.08911757 1.936508 2.020049 1.4654399 2.563985
## 14 1.328742 0.20749565 1.298485 1.333454 0.7804978 1.876987
## 19 1.534162 0.08986811 1.522672 1.550848 0.9859820 2.082341
spectratrait::f.plot.coef(Z = t(Jackknife_coef), wv = wv,
plot_label="Jackknife regression coefficients",position = 'bottomleft')
abline(h=0,lty=2,col="grey50")
box(lwd=2.2)
dev.copy(png,file.path(outdir,paste0(inVar,'_Jackknife_Regression_Coefficients.png')),
height=2100, width=3800, res=340)## quartz_off_screen
## 3
dev.off();## quartz_off_screen
## 2
rmsep_percrmsep <- spectratrait::percent_rmse(plsr_dataset = val.plsr.output,
inVar = inVar,
residuals = val.plsr.output$PLSR_Residuals,
range="full")
RMSEP <- rmsep_percrmsep$rmse
perc_RMSEP <- rmsep_percrmsep$perc_rmse
r2 <- round(pls::R2(plsr.out, newdata = val.plsr.data,intercept=F)$val[nComps],2)
expr <- vector("expression", 3)
expr[[1]] <- bquote(R^2==.(r2))
expr[[2]] <- bquote(RMSEP==.(round(RMSEP,2)))
expr[[3]] <- bquote("%RMSEP"==.(round(perc_RMSEP,2)))
rng_vals <- c(min(val.plsr.output$LPI), max(val.plsr.output$UPI))
par(mfrow=c(1,1), mar=c(4.2,5.3,1,0.4), oma=c(0, 0.1, 0, 0.2))
plotrix::plotCI(val.plsr.output$PLSR_Predicted,val.plsr.output[,inVar],
li=val.plsr.output$LPI, ui=val.plsr.output$UPI, gap=0.009,sfrac=0.004,
lwd=1.6, xlim=c(rng_vals[1], rng_vals[2]), ylim=c(rng_vals[1], rng_vals[2]),
err="x", pch=21, col="black", pt.bg=scales::alpha("grey70",0.7), scol="grey50",
cex=2, xlab=paste0("Predicted ", paste(inVar), " (units)"),
ylab=paste0("Observed ", paste(inVar), " (units)"),
cex.axis=1.5,cex.lab=1.8)
abline(0,1,lty=2,lw=2)
legend("topleft", legend=expr, bty="n", cex=1.5)
box(lwd=2.2)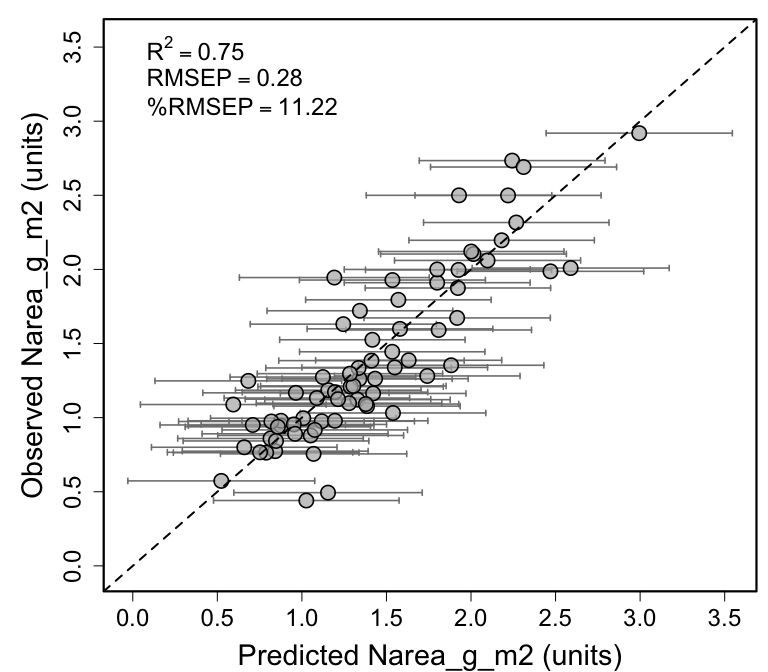
dev.copy(png,file.path(outdir,paste0(inVar,"_PLSR_Validation_Scatterplot.png")),
height=2800, width=3200, res=340)## quartz_off_screen
## 3
dev.off();## quartz_off_screen
## 2
out.jk.coefs <- data.frame(Iteration=seq(1,length(Jackknife_intercept),1),
Intercept=Jackknife_intercept,t(Jackknife_coef))
head(out.jk.coefs)[1:6]## Iteration Intercept Wave_500 Wave_501 Wave_502 Wave_503
## Seg 1 1 -0.001089661 0.3156927 0.3524556 0.3947195 0.4329382
## Seg 2 2 0.082969588 0.2989509 0.3382983 0.3835509 0.4239103
## Seg 3 3 0.114879574 0.2716867 0.3122469 0.3574386 0.3982935
## Seg 4 4 0.178884696 0.2099486 0.2520760 0.3018899 0.3452178
## Seg 5 5 0.126339690 0.2898707 0.3311239 0.3762377 0.4163999
## Seg 6 6 -0.085381533 0.2805890 0.3195387 0.3625074 0.4023830
write.csv(out.jk.coefs,file=file.path(outdir,
paste0(inVar,
'_Jackkife_PLSR_Coefficients.csv')),
row.names=FALSE)print(paste("Output directory: ", outdir))## [1] "Output directory: /var/folders/th/fpt_z3417gn8xgply92pvy6r0000gq/T//RtmpP2TT60"
# Observed versus predicted
write.csv(cal.plsr.output,file=file.path(outdir,
paste0(inVar,'_Observed_PLSR_CV_Pred_',
nComps,'comp.csv')),
row.names=FALSE)
# Validation data
write.csv(val.plsr.output,file=file.path(outdir,
paste0(inVar,'_Validation_PLSR_Pred_',
nComps,'comp.csv')),
row.names=FALSE)
# Model coefficients
coefs <- coef(plsr.out,ncomp=nComps,intercept=TRUE)
write.csv(coefs,file=file.path(outdir,
paste0(inVar,'_PLSR_Coefficients_',
nComps,'comp.csv')),
row.names=TRUE)
# PLSR VIP
write.csv(vips,file=file.path(outdir,
paste0(inVar,'_PLSR_VIPs_',
nComps,'comp.csv')))print("**** PLSR output files: ")## [1] "**** PLSR output files: "
print(list.files(outdir)[grep(pattern = inVar, list.files(outdir))])## [1] "Narea_g_m2_Cal_PLSR_Dataset.csv"
## [2] "Narea_g_m2_Cal_Val_Histograms.png"
## [3] "Narea_g_m2_Cal_Val_Scatterplots.png"
## [4] "Narea_g_m2_Cal_Val_Spectra.png"
## [5] "Narea_g_m2_Coefficient_VIP_plot.png"
## [6] "Narea_g_m2_Jackkife_PLSR_Coefficients.csv"
## [7] "Narea_g_m2_Jackknife_Regression_Coefficients.png"
## [8] "Narea_g_m2_Observed_PLSR_CV_Pred_10comp.csv"
## [9] "Narea_g_m2_PLSR_Coefficients_10comp.csv"
## [10] "Narea_g_m2_PLSR_Component_Selection.png"
## [11] "Narea_g_m2_PLSR_Validation_Scatterplot.png"
## [12] "Narea_g_m2_PLSR_VIPs_10comp.csv"
## [13] "Narea_g_m2_Val_PLSR_Dataset.csv"
## [14] "Narea_g_m2_Validation_PLSR_Pred_10comp.csv"
## [15] "Narea_g_m2_Validation_RMSEP_R2_by_Component.png"